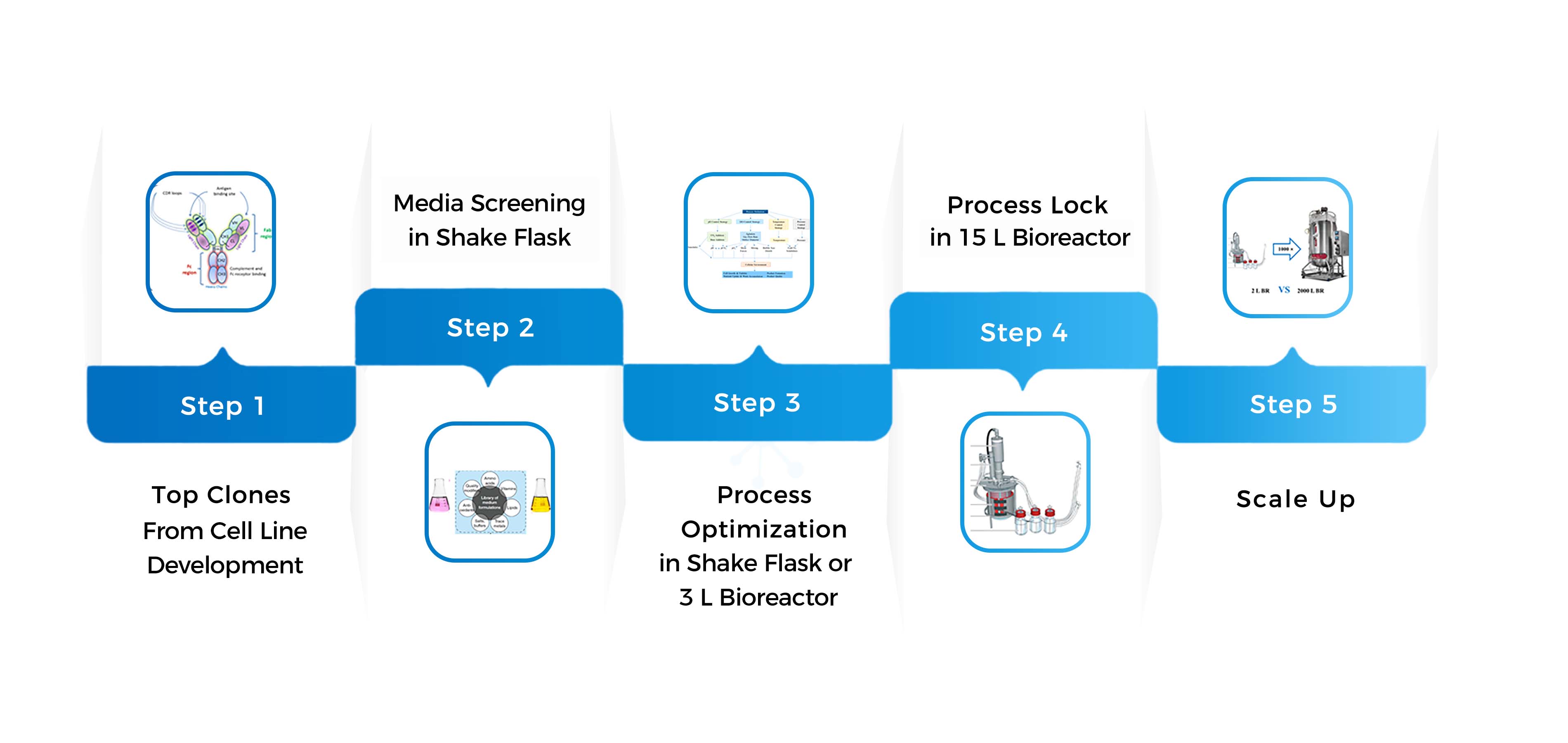
In early clinical phases, speed, flexibility, and repeatability are critical for drug substance process development.
Our in-house experts maximize batch yield and reduce processing time for your molecule while developing an optimal process with long-term commercial manufacturing in mind.